Plant responses to environmental signals and phytohormones
In order to grow, plants need to respond to ever-changing environmental signals, including light, water status, nutrients, and temperature. In response to such environmental signals, stomatal pores regulate water loss from leaves and uptake of CO2, for use in photosynthesis (Fig. 1). 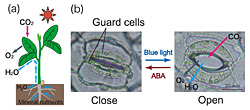 Each stomatal pore is surrounded by a pair of guard cells; stomatal opening is driven by the accumulation of K+ salts in guard cells, which is mediated by a blue light-activated electrogenic proton pump in the plasma membrane. Recently, we have shown that phototropins (phot1 and phot2), which are light receptor-type Ser/Thr protein kinases, serve as redundant blue light receptors for stomatal opening (1, 2). Furthermore, blue light activates the plasma membrane proton pump (H+-ATPase) via the phosphorylation of a penultimate threonine in the C-terminus and subsequent binding of the 14-3-3 protein to the phosphorylated C-terminus (3) (Fig. 2).
Each stomatal pore is surrounded by a pair of guard cells; stomatal opening is driven by the accumulation of K+ salts in guard cells, which is mediated by a blue light-activated electrogenic proton pump in the plasma membrane. Recently, we have shown that phototropins (phot1 and phot2), which are light receptor-type Ser/Thr protein kinases, serve as redundant blue light receptors for stomatal opening (1, 2). Furthermore, blue light activates the plasma membrane proton pump (H+-ATPase) via the phosphorylation of a penultimate threonine in the C-terminus and subsequent binding of the 14-3-3 protein to the phosphorylated C-terminus (3) (Fig. 2).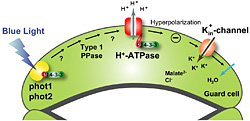 Fig. 2. Schematic model of blue-light signaling pathway in stomatal guard cells.
In contrast, stomata close in response to the phytohormone abscisic acid (ABA), in order to prevent water loss. ABA-signaling pathways have been extensively investigated in the hopes of understanding the molecular mechanism of the plant response to drought stress. We have already shown that ABA-induced phosphorylation plays an important role in ABA-signaling in guard cells (4). However, the signaling mechanisms that control stomatal opening and closing have yet to be determined.
Our aim is to elucidate signaling pathways in stomatal guard cells in response to light and phytohormones, and the molecular mechanism of regulation of the plasma membrane H+-ATPase. We are currently studying the signaling pathways that control stomatal opening and closing using genetic, physiological and biochemical approaches; we are focusing on the biochemical properties of the phosphorylation reaction and the H+-ATPase complex (5). In addition, we are also interested in plant growth and development, especially in response to light and phytohormones such as auxin and brassinosteroid (6).
Fig. 2. Schematic model of blue-light signaling pathway in stomatal guard cells.
In contrast, stomata close in response to the phytohormone abscisic acid (ABA), in order to prevent water loss. ABA-signaling pathways have been extensively investigated in the hopes of understanding the molecular mechanism of the plant response to drought stress. We have already shown that ABA-induced phosphorylation plays an important role in ABA-signaling in guard cells (4). However, the signaling mechanisms that control stomatal opening and closing have yet to be determined.
Our aim is to elucidate signaling pathways in stomatal guard cells in response to light and phytohormones, and the molecular mechanism of regulation of the plasma membrane H+-ATPase. We are currently studying the signaling pathways that control stomatal opening and closing using genetic, physiological and biochemical approaches; we are focusing on the biochemical properties of the phosphorylation reaction and the H+-ATPase complex (5). In addition, we are also interested in plant growth and development, especially in response to light and phytohormones such as auxin and brassinosteroid (6).
Cell wall function
The elongation growth of plants is caused by a remarkable cell enlargement along the axial direction, which is a consequence of water absorption and cell wall extension. Auxin, a phytohormone that promotes elongation growth, is thought to stimulate proton extrusion by the plasma membrane H+-ATPases, resulting in acidification of the apoplastic space and induction of wall extension (the "acid growth" hypothesis). Recently, the acid growth hypothesis has been reinforced by the discovery of a protein, expansin, which induces wall elongation (wall-loosening) under acidic condition. So far, we have shown that expansins regulate wall yield threshold tension as well as wall extensibility (7, 8). We are now studying the role of expansins on the elongation growth, using Arabidopsis mutants. Our goal is elucidation of the molecular mechanism of cell wall extension.
Cell wall structures are also found in prokaryotes, fungi and (micro)algae; animal immune systems utilize their cell wall components in order to sense invasion of these organisms. When host immune cells recognize these cell wall components, they commence an immune response to eliminate the organisms. We are also investigating the participation of cell wall polysaccharides in these host reactions.


References
- Kinoshita et al. (2001) phot1 and phot2 mediate blue light regulation of stomatal opening. Nature 414: 656-660.
- Inoue et al. (2008) Blue light-induced autophoshporylation of phototropin is a primary step for signaling. Proc. Natl. Acad. Sci. USA 105: 5626-5631.
- Kinoshita and Shimazaki (1999) Blue light activates the plasma membrane H+-ATPase by phosphorylation of the C-terminus in stomatal guard cells. EMBO J. 18: 5548-5558.
- Li et al. (2002) Modulation of an RNA-binding protein by abscisic-acid-activated protein kinase. Nature 418: 793-797.
- Hayashi et al. (2010) Biochemical characterization of in vitro phosphorylation and dephosphorylation of the plasma membrane H+-ATPase. Plant Cell Physiol. 51: 1186-1196.
- Kinoshita et al. (2005) Binding of brassinosteroids to the extracellular domain of plant receptor kinase BRI1. Nature 433: 167-171.
- Ezaki et al. (2005) The role of wall Ca2+ in the regulation of wall extensibility during the acid-induced extension of soybean hypocotyl cell walls. Plant Cell Physiol. 46: 1831-1838.
- Takahashi et al. (2006) Wall-yielding properties of cell walls from elongating cucumber hypocotyls in relation to the action of expansin. Plant Cell Physiol. 47: 1520-1529.